Pillars for prevention and control of healthcare-associated infections: an Italian expert opinion statement
Healthcare-associated infections (HAIs) represent a relevant problem for all healthcare facilities, because they involve both the care aspect and the economic management of the hospital. Most HAIs are preventable through effective Infection Prevention and Control (IPC) measures. Implementation and improvement of IPC programs are critical to reducing the impact of these infections and the spread of multi-resistant microorganisms. The purpose of this Expert Opinion statement was to provide a practical guide for healthcare organizations, physicians, and nursing staff on the optimal implementation of the core components of Infection Prevention and Control, as recommended by a board of specialists after in-depth discussion of the available evidence in this field. According to their independent suggestions and clinical experiences, as well as evidence-based practices and literature review, this document provides a practical bundle of organizational, structural, and professional requirements necessary to promote, through multimodal strategies, the improvement of the quality and safety of care with respect to infectious risk in order to protect the patient, facilities, and healthcare providers.
Introduction
Healthcare-associated infections (HAIs) are a major public health problem both because of the significant impact on patient morbidity, mortality, and quality of life and because of the significant economic burden on healthcare systems worldwide. However, most HAIs are preventable and can be reduced by up to 70% through effective Infection Prevention and Control (IPC) measures [1].
Improvements in IPC at the national and facility level are critical to the containment of antimicrobial resistance and prevention of HAIs, including outbreaks of highly transmissible diseases, through high-quality care in the context of universal health coverage [2].
In 2016, World Health Organization (WHO) published recommendations on effective IPC strategies based on systematic literature review and expert consensus summarized in eight core components, followed by a set of minimum requirements for their implementation [3,4,5]. According to these documents, the core components needed to improve IPC practices are: (1) IPC programme, (2) IPC guidelines, (3) IPC education and training, (4) Healthcare-associated infection surveillance, (5) Multimodal strategies, (6) Monitoring/audit of IPC practices and feedback, (7) Workload, staffing and bed occupancy and (8) Built environment, materials and equipment for IPC.
In 2017, an IPC assessment framework (IPCAF) was developed by the WHO IPC Global Unit to support the implementation of WHO guidelines on core components of IPC programs in acute healthcare facilities and to enable these facilities to self-evaluate IPC practices [6]. However, applying the 2017 IPCAF, several surveys have shown that the WHO core components are difficult to implement at the facility level [7,8,9].
A board of specialists agreed to draft an expert opinion paper to guide healthcare organizations, physicians, and nurses on the optimal implementation of the HAI-IPC core components, after a thorough discussion of the available evidence in this field.
The purpose of this document is to provide healthcare organisations with a practical bundle of organisational, structural and professional requirements necessary to promote, through multimodal strategies, the improvement of the quality and safety of care with regard to infectious risk, in order to protect the patient, facilities, and healthcare workers.
Methods
Prevention and management of HAIs should focus on collaboration among all healthcare professionals with shared knowledge and widespread diffusion of best practices.
Seven different Italian professionals involved in the issue of HAIs prevention and control in their respective hospitals convened in a panel in order to identify and provide a practical set of organizational, structural, and professional requirements crucial for effective implementation of infection control programs: three infectious disease specialists, two nurses, a Director of microbiology, and a Director of emergency and trauma surgery with a well-known experience in surgical infections and sepsis.
The experts decided to draft an expert opinion paper on the prevention, surveillance, and control of HAIs in healthcare facilities, according to their independent suggestions and clinical experiences, as well as to evidence-based practices. A non-systematic review of the literature was performed and discussed by the panel.
During a first virtual meeting in May 2021, the experts outlined the HAI risk scenario and identified risk factors through a series of questions (brainstorming) to discuss the main areas of interest with the aim of producing a document as a useful support tool for all healthcare professionals working in the context of HAI prevention and monitoring processes. All feedback from the brainstorming was collected and shared via email prior to the second virtual meeting held in July 2021 where the experts wrote the outline of the paper. The experts communicated via email to prepare, discuss, and revise the document, adding boxes to the paper in which to describe some personal experiences or common practices of their hospitals.
Referring to the core components of the WHO IPC programs, this document was set up along three dimensions, addressed separately:
- (1) Organisational and structural arrangements to implement programs of IPC
- (2) Targets and methods of HAI surveillance, monitoring and role of feedback, in the context of multimodal prevention strategies
- (3) Standard Operating Procedures (SOP), methods and effectiveness of healthcare workers educating and training, and interventions on behavioural change and quality of care
Dimensions 2 and 3 were contextualized according to the multimodal prevention strategies. The panel did not take into account the role of antimicrobial stewardship in controlling AMR although recognizing it a fundamental tool and thus referring to other documents [10,11,12,13].
Background
Epidemiology
HAIs affect millions of patients worldwide every year. The total annual number of patients with an HAI in European acute care hospitals in 2011–2012 was estimated at 3.2 million, causing 37,000 deaths as a direct consequence, more than 2.5 million Disability Adjusted Life Years (DALYs) and 16 million extra days of hospitalization, with an approximate cost of around €7 billion [14].
The 2016–2017 Point Prevalence Survey (PPS) by the European Centre for Disease Prevention and Control (ECDC) has estimated that 8.9 million distinct HAI episodes occur annually in acute care hospitals and long-term care facilities in Europe [15].
In Italy, a number between 450,000 and 700,000 cases of HAI is estimated to occur every year in hospitalized patients, of which 30% considered preventable [16]. The 2016/2017 Italian PPS2 report on HAIs and antibiotic use in acute care hospitals reported that the prevalence of patients with at least one HAI was 8.03%, whereas when considering the average prevalence of HAIs across hospitals, this estimate becomes 6.5%, indicating that some larger, highly specialized hospitals showed a higher prevalence [17,18,19,20].
Hospital-acquired infection sites
The most frequent types of infection include.
- 1. Catheter-Associated Urinary Tract Infections (CAUTI).
- 2. Surgical Site Infections (SSI).
- 3. Hospital-Acquired Pneumonia (HAP) and Ventilator Associated Pneumonia (VAP).
- 4. Central Line-Associated BloodstreamIinfections (CLABSI).
- 5. Care-related Skin and Soft Tissue Infections (SSTI).
A brief detail of these is given below:
Catheter associated urinary tract infections (CAUTI)
Among hospital-acquired urinary tract infections, 70–80% are attributable to the use of an indwelling urinary catheter. Catheter-Associated Urinary Tract Infections (CAUTI) are the most frequently observed conditions, with an incidence 40% of all HAIs [21].
The duration of catheterization is the most important risk factor for developing CAUTI. Therefore, reducing unnecessary catheter placement and minimizing the duration of catheter stay in situ are the main strategies for prevention of CAUTI. Additional risk factors include female sex, older age, diabetes mellitus, renal failure, and malnutrition [22].
Although the proportion of bacteremic individuals who develop symptomatic infection is low, given the high frequency of indwelling urinary catheter use, CAUTI is one of the most common causes of secondary bloodstream infection. CAUTI is the source of approximately 20% of healthcare-acquired bacteraemia episodes in acute care facilities and more than 50% in long-term care facilities [23].
The estimated cost of HAIs in a Polish Intensive Care Unit (ICU) ranges from EUR 10,035 to 22,411. While in the USA, an estimate of 449,334 healthcare-associated CAUTIs per year, is associated with an additional cost of US$749–10,077-9 per admission in 2007 (or an estimated US$3744 when complicated by blood septicaemia) [24].
Comprehensive recommendations have been published to assist acute care hospitals in implementing and prioritizing their CAUTI prevention efforts [25,26,27].
Surgical site infections (SSI)
Surgical Site Infections (SSIs) are a major complication during hospitalization, occurring in 2–5% of patients subjected to surgery. These are the second most common type of nosocomial infections caused primarily by Staphylococcus aureus resulting in prolonged hospitalization and increased risk of mortality. In most SSIs, the responsible pathogens originate from the patient’s endogenous flora [28].
From the 2017 European Annual Epidemiology Report, the percentage of SSIs ranges from 0.5% to 10.1%, depending on the type of surgical procedure [29].
SSIs are defined as infections occurring up to 30 days after surgery (if no implant is left in place) and affecting either the incision or deep tissue at the operation site. These infections may be superficial involving only skin and subcutaneous tissue of the incision, or deep incisional infections down to the deep soft tissues (fascia and muscle), or infections involving organs and body spaces.
Major patient-related risk factors include advanced age, diabetes mellitus or other chronic diseases, nutritional status, obesity, immunodepression, colonisation with microorganisms (particularly S. aureus). Emergency surgery, type of surgery, length and quality of preoperative stay, skin disinfection, inadequate sterilization of surgical instruments and antimicrobial prophylaxis are procedure-related risk factors [30].
Thus, continuous vigilance is required to minimize the incidence of such infections. This requires a systematic approach, with attention to multiple risk factors to reduce the risk of bacterial contamination and improve patient’s defences [31, 32].
The 2017 Centres for Disease Control and Prevention (CDC) guideline provides new and updated evidence-based recommendations for the prevention of SSI and should be incorporated into comprehensive surgical quality improvement programs to improve patient safety [33, 34].
SSIs are responsible for generating significant costs. In 2017, a French cohort showed an average cost of each SSI treatment of approximately €1814; the same year, the Centres for Disease Control and Prevention guidelines estimated the mean cost caused by SSI treatment at $10,443–$25,546 per SSI. This cost depends on many factors including the patient themselves and the type of surgery [35].
Hospital-acquired pneumonia (HAP) and Ventilator associated pneumonia (VAP)
Hospital-Acquired Pneumonia (HAP) is the second most common infectious complication contracted during hospitalization. The incidence ranges from 5 to 10 cases per 1000 admissions in patients without risk factors, but this estimate may increase can increase 6 to 20-fold in patients admitted to ICU and receiving mechanical ventilation [36].
Ventilator Associated Pneumonia (VAP) is found in 9–27% of mechanically ventilated patients and usually occurs within 48 h after tracheal incubation [37]. The risk of contracting VAP increases proportionally with prolonging duration of both mechanical ventilation and ICU stay. Mortality attributable to VAP ranges from 15 to 50%, with higher mortality rates in surgical patients in the ICU and in patients with average severity scores on admission [38, 39].
The results of a study on the effect of VAP on the prognosis of ICU patients within 90 days and 180 days showed that the 90-day mortality of VAP patients was 33.33% and the 180-day mortality was 37.62%. The 90-day and 180-day mortality rates were higher in the VAP group than in the non-VAP group. The risk of 90-day and 180-day mortalities in VAP patients were 1.465 times (OR = 1.465, 95% CI: 1.188–1.807, P < 0.001) and 1.635 times (OR = 1.635, 95% CI: 1.333–2.005, P < 0.001) higher than those in non-VAP patients, respectively [40].
New international evidence-based guidelines for the prevention of HAP/VAP have recently been published in Europe and America and provide guidance on the most effective treatments and management strategies for adult patients with HAP and VAP [36, 41].
Central line-associated bloodstream infections (CLABSI)
The main source of infections in the circulatory stream are due to the implantation of vascular catheters (Central Line-Associated Bloodstream Infection—CLABSI). Catheters are placed in the central line to deliver fluids and medications, but prolonged use can cause severe bloodstream infections resulting in impaired health, and increased hospitalization and cost of care [42]. CLABSIs account for approximately 20% of nosocomial circulatory infections, with a mortality rate of 12–25% [43].
Host factors that increase the risk of CLABSI are chronic disease, immunocompromised states (organ transplantation, diabetes mellitus), malnutrition, total parenteral nutrition, loss of skin integrity (burns), and prolonged hospitalization prior to catheter insertion. Femoral central venous catheters are associated with the highest risk of CLABSI, followed by internal jugular and subclavian catheters. In addition, catheter type, insertion conditions, catheter care, and operator skill also influence the risk of CLABSI.
Of all HAIs, CLABSIs are associated with a high-cost burden of approximately $46,000 per case [44]. Most cases are preventable with appropriate aseptic techniques, surveillance, and management strategies.
The updated guideline released by the CDC in 2011 along with more recent studies highlight new strategies to reduce the incidence of CLABSI [45–47].
Care-related skin and soft tissue infections (SSTI)
Skin and Soft Tissue Infections (SSTIs) are common in outpatient clinic and emergency department visits and include a wide variety of infections of the various layers of skin, fascia, and muscle. SSTIs usually result from traumatic, surgical, or healthcare-related skin breakdown with secondary infection by microorganisms [48].
The severity of SSTIs ranges from mild and superficial to deeper or potentially fatal necrotizing infections requiring hospitalization or intensive care. Among hospitalized or critically ill patients, several epidemiological studies have shown that about 4.3–10.5% of septic episodes are caused by SSTIs [49, 50].
Using data from the 2000–2004 US Healthcare Cost and Utilization Project National Inpatient Sample, Edelsberg et al. suggested that the majority of hospitalized SSTIs were either “superficial” (58.6%) or “deeper and/or healthcare-associated” (40.1%) infections; the percentage of “often fatal” SSTIs was relatively low (1.3%) [51].
In a large database study of skin-related conditions in the ICU, only 0.4% of all ICU admissions had SSTIs, and about 60% of those were necrotizing fasciitis, a potentially fatal infection [52].
Two other studies, including only “superficial” and “deep and/or healthcare-associated” infections, showed that approximately 2.0–5.8% of patients hospitalized with SSTIs are admitted to the ICU [53, 54].
A large number of expert opinions, guidelines, and recommendations for the management of SSTIs have been published over the past decade, taking into account the initial severity of the patient (whether or not the patient requires ICU admission), the extent of the infection (superficial or deep infection), and risk factors for resistant microorganisms essentially related to healthcare-associated circumstances [55,56,57,58].
Factors influencing the development of HAIs
Numerous factors can increase the risk of contracting a HAI, which, in general, can be divided into three groups: host factors, microorganism-related factors, and environmental factors [59].
Patient-related risk factors include advanced age, multiple underlying comorbidities, chronic conditions such as chronic kidney disease, cardio-respiratory disease, and diabetes mellitus, and all conditions of immunosuppression related to drugs (steroid therapy, chemotherapy, radiation therapy, immunomodulatory therapies) and diseases (HIV infection, onco-hematologic diseases, solid organ or bone marrow transplantation, burns, and malnutrition) [60].
Potentially all microorganisms can cause HAI, but generally bacteria and viruses are the main infectious agents. One of the main risk factors related to microorganisms is certainly antibiotic resistance [61].
Finally, the main environmental risk factors are exposure to invasive procedures that increase the risk of infectious complications due to direct access of microorganisms to normally sterile areas of the body and contamination of the devices themselves at the time of use. Exposure to invasive procedures and/or complex surgeries, length of hospital stay, frequent visits to healthcare facilities, mechanical ventilator support, and a stay in an ICU also increase the likelihood of infectious complications [62].
In conclusion, the risk of HAIs depends on the infection control practices in the facility, the condition and immune status of the patient, and the prevalence of various pathogens in the community.
Mode of transmission
The main route of transmission is by contact. Direct or indirect contact and transmission by droplets fall into this category [63].
Direct contact between a susceptible host and an infected or colonized person can occur during daily care activities, primarily through hands, or through the contact between patients.
Indirect contact occurs through a contaminated intermediate object (instrumentation/surfaces) or through a contaminated common vehicle (food, blood, infusion fluids, disinfectants).
Droplet transmission occurs when droplets containing the pathogens emitted in the act of coughing or sneezing by an infected person are inhaled by a susceptible person who is within a short distance (via droplets) or at a distance (aerosol). Another mode of transmission is by the airborne route, through microorganisms that survive in the air and are transmitted over distance.
Discussion
The expert panel identified a range of structural, organizational, and management components, given the existing evidence and experts’ opinions, that are crucial to effective implementation of infection control programmes in a hospital setting.
Core component 1: IPC ORGANIZATION, STRUCTURE
Organisational and structural arrangements to implement programs of IPC
Facility’s top management should clearly express the healthcare facility's commitment to address HAIs and antimicrobial resistance (AMR) in a shared and signed document, defining the budget, allocating resources (bed occupancy, staffing, workload, materials and equipment) and how it intends to pursue it in a programme (objectives, activities, timeline, indicators) [62].
As an example, Box 1 shows the annual plan for prevention and control of HAIs of the “Alessandro Manzoni” Hospital in Lecco, Italy.
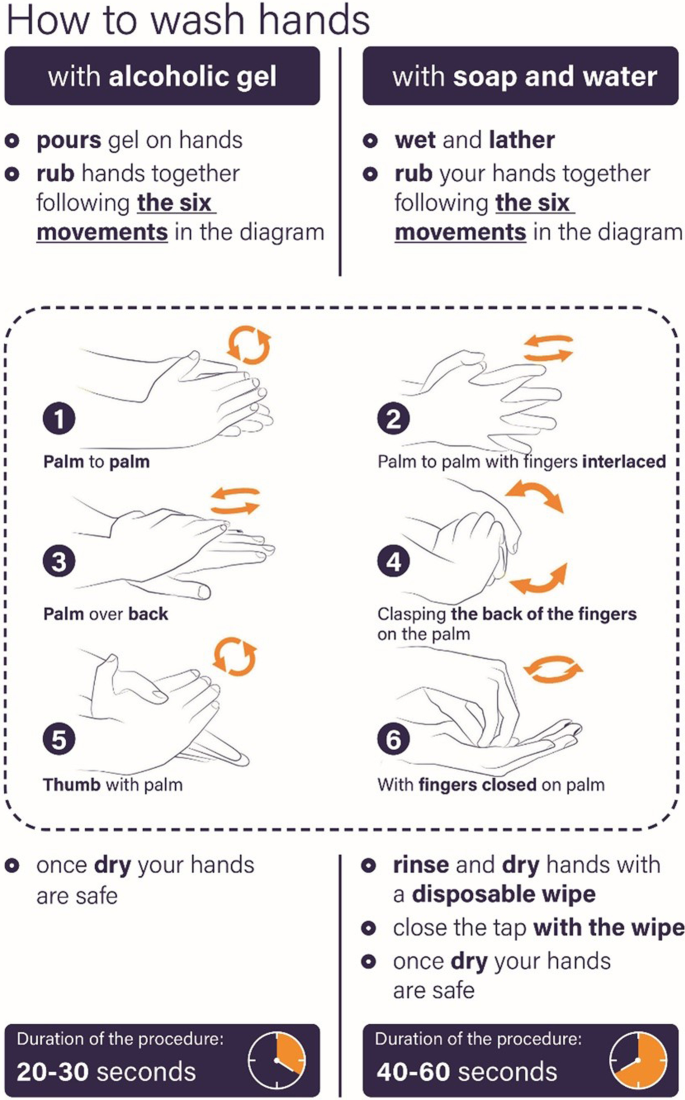
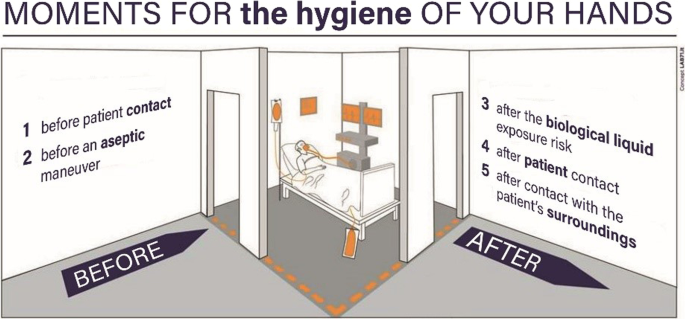
Conclusion
Implementation of the WHO recommendations on the "core components" of IPC is necessary to build functioning programs that lead to the effective reduction of HAIs.
Referring to the core components of the WHO IPC programs, this expert opinion statement was set up along three dimensions, addressed separately: (1) organizational and structural arrangements to implement IPC programs with clearly defined objectives, functions, and activities for the purpose of preventing HAIs through IPC good practices; (2) targets and methods of HAI surveillance, monitoring, outbreak management, and role of feedback; (3) methods and effectiveness of healthcare workers educating and training.
As a result of the activities and actions taken by some Italian hospitals described in the boxes, for example, a more appropriate use of antibiotics has been achieved, leading to a decrease in in-hospital use of fluoroquinolones and carbapenems; good practice of hand hygiene has increased, reaching an overall compliance of more than 80%; and a specific protocol on laboratory-based surveillance of MDR alert organisms has been developed, leading to better management of MDR carriers.
In conclusion, sharing independent suggestions on an effective risk management plan and comparing clinical experiences can be helpful in identifying interventions and actions needed to monitor and contain HAIs.
It is important to clarify that this document is based only on Italian experiences and practices in HAI prevention and control and cannot represent universal recommendations. However, the interventions and strategies proposed here to improve the quality and safety of care with respect to infectious risk can be applied to other countries, including those with low resources.
Availability of data and materials
Abbreviations
Catheter-Associated Urinary Tract Infection
Centres for Disease Control and Prevention
Central Line-Associated Bloodstream Infection
Carbapenemase-producing Enterobacterales
Carbapenem-resistant Acinetobacter baumannii
Carbapenem-resistant Enterobacterales
European Centre for Disease Prevention and Control
Intensive Care Unit
Infection Prevention and Control
Piano Annuale delle Infezioni Correlate all’Assistenza (Annual Plan of Healthcare-Associated Infections)
National Plan to Combat Antimicrobial Resistance
Point Prevalence Survey
Standard Operating Procedures
Surgical Site Infection
Skin and Soft Tissue Infection
Ventilator Associated Pneumonia
Whole Genome Sequencing
World Health Organization
References
- Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101–14. ArticlePubMedGoogle Scholar
- Zingg W, Holmes A, Dettenkofer M, Goetting T, Secci F, Clack L, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015;15(2):212–24. ArticlePubMedGoogle Scholar
- World Health Organization. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. Geneva: WHO; 2016. Available at: https://www.who.int/gpsc/core-components.pdf
- World Health Organization. Minimum requirements for infection prevention and control programmes. Geneva: WHO; 2019. Available at: https://www.who.int/infection-prevention/publications/MinReq-Manual_2019.pdf?ua=1
- Storr J, Twyman A, Zingg W, Damani N, Kilpatrick C, Reilly J, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6:6. ArticlePubMedPubMed CentralGoogle Scholar
- Tomczyk S, Aghdassi S, Storr J, Hansen S, Stewardson AJ, Bischoff P, et al. Testing of the WHO infection prevention and control assessment framework at acute healthcare facility level. J Hosp Infect. 2020;105(1):83–90. ArticleCASPubMedGoogle Scholar
- Tartari E, Tomczyk S, Pires D, Zayed B, Coutinho Rehse AP, Kariyo P, et al. Implementation of the infection prevention and control core components at the national level: a global situational analysis. J Hosp Infect. 2021;108:94–103. ArticleCASPubMedPubMed CentralGoogle Scholar
- Aghdassi SJS, Grisold A, Wechsler-Fördös A, Hansen S, Bischoff P, Behnke M, Gastmeier P. Evaluating infection prevention and control programs in Austrian acute care hospitals using the WHO Infection Prevention and Control Assessment Framework. Antimicrob Resist Infect Control. 2020;9(1):92. ArticlePubMedPubMed CentralGoogle Scholar
- Aghdassi SJS, Hansen S, Bischoff P, Behnke M, Gastmeier P. A national survey on the implementation of key infection prevention and control structures in German hospitals: results from 736 hospitals conducting the WHO Infection Prevention and Control Assessment Framework (IPCAF). Antimicrob Resist Infect Control. 2019;8:73. ArticlePubMedPubMed CentralGoogle Scholar
- Onorato L, Macera M, Calò F, Monari C, Russo F, Iovene MR, Signoriello G, Annibale R, Pace MC, Aurilio C, Gaeta GB, Coppola N. The effect of an antimicrobial stewardship programme in two intensive care units of a teaching hospital: an interrupted time series analysis. Clin Microbiol Infect. 2020;26(6):782.e1-e6. ArticleCASGoogle Scholar
- Hulscher MEJL, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect. 2017;23(11):799–805. ArticleCASPubMedGoogle Scholar
- Centers for Disease Control and Prevention. The Core Elements of Hospital Antibiotic Stewardship Programs: 2019. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
- World Health Organization 2021. Antimicrobial stewardship interventions: a practical guide. Available at: https://apps.who.int/iris/bitstream/handle/10665/340709/9789289054980-eng.pdf
- European Centre for Disease Prevention and Control (ECDC) SURVEILLANCE REPORT. Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. 2013. Available at: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):1800516. ArticlePubMed CentralGoogle Scholar
- Ministry of Health. Igiene delle mani può ridurre del 30% le infezioni ospedaliere. 2019. Available at: https://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=null&id=3736
- REPORT ITALIANO PPS2 2016/2017. Studio di prevalenza italiano sulle infezioni correlate all’assistenza e sull’uso di antibiotici negli ospedali per acuti – protocollo ECDC. Available at: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2791_allegato.pdf
- Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank H-P, Ducomble T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based Modelling study. PLOS Med. 2016;13(10): e1002150. ArticlePubMedPubMed CentralGoogle Scholar
- Istituto Superiore di Sanita. Infezioni correlate all’assistenza aspetti epidemiologici [Istituto Superiore di Sanità - Healthcare Associated Infections, epidemiological aspects] Available at: https://www.epicentro.iss.it/infezioni-correlate/epidemiologia
- Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–41. ArticlePubMedGoogle Scholar
- Magill SS, Edwards JR, Bamberg W, Beldaus ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. ArticleCASPubMedPubMed CentralGoogle Scholar
- Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. ArticlePubMedPubMed CentralGoogle Scholar
- Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 2012;17(46):20316. ArticlePubMedGoogle Scholar
- Gad MH, AbdelAziz HH. Catheter-associated urinary tract infections in the Adult Patient Group: a qualitative systematic review on the adopted preventative and interventional protocols from the literature. Cureus. 2021;13(7): e16284. PubMedPubMed CentralGoogle Scholar
- Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464–79. ArticlePubMedGoogle Scholar
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–63. ArticlePubMedGoogle Scholar
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–26. ArticlePubMedGoogle Scholar
- Anderson DJ. Surgical site infections. Infect Dis Clin North Am. 2011;25(1):135–53. ArticlePubMedGoogle Scholar
- European Centre for Disease Prevention and Control. Healthcare-associated infections: surgical site infections. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SSI.pdf
- Owens CD. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008;70(Suppl 2):3–10. ArticlePubMedGoogle Scholar
- Sganga G, Tascini C, Sozio E, Colizza S. Early recognition of methicillin-resistant Staphylococcus aureus surgical site infections using risk and protective factors identified by a group of Italian surgeons through Delphi method. World J Emerg Surg. 2017;12:25. ArticleCASPubMedPubMed CentralGoogle Scholar
- Sganga G, Tascini C, Sozio E, Carlini M, Chirletti P, Cortese F, et al. Focus on the prophylaxis, epidemiology and therapy of methicillin-resistant Staphylococcus aureus surgical site infections and a position paper on associated risk factors: the perspective of an Italian group of surgeons. World J Emerg Surg. 2016;11:26. ArticleCASPubMedPubMed CentralGoogle Scholar
- Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–91. ArticlePubMedGoogle Scholar
- O’Hara LM, Thom KA, Preas MA. Update to the centers for disease control and prevention and the healthcare infection control practices advisory committee guideline for the prevention of surgical site infection (2017): a summary, review, and strategies for implementation. Am J Infect Control. 2018;46(6):602–9. ArticlePubMedGoogle Scholar
- Piednoir E, Robert-Yap J, Baillet P, Lermite E, Christou N. The Socioeconomic impact of surgical site infections. Front Public Health. 2021;9:712461. ArticlePubMedPubMed CentralGoogle Scholar
- Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50(3):1700582. ArticlePubMedCASGoogle Scholar
- Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344: e3325. ArticlePubMedGoogle Scholar
- Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. ArticlePubMedPubMed CentralGoogle Scholar
- Steven M, Koenig JDT. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637–57. ArticleGoogle Scholar
- Luo W, Xing R, Wang C. The effect of ventilator-associated pneumonia on the prognosis of intensive care unit patients within 90 days and 180 days. BMC Infect Dis. 2021;21(1):684. ArticlePubMedPubMed CentralGoogle Scholar
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. ArticlePubMedPubMed CentralGoogle Scholar
- Bell T, O’Grady NP. Prevention of central line-associated bloodstream infections. Infect Dis Clin North Am. 2017;31(3):551–9. ArticlePubMedPubMed CentralGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Vital signs: Central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011; 60(8):243–8.
- Haddadin Y, Annamaraju P, Regunath H. Central Line Associated Blood Stream Infections. StatPearls [Internet] 2021. Treasure Island (FL). PMID: 28613641.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–93. ArticlePubMedPubMed CentralGoogle Scholar
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32. ArticleCASPubMedGoogle Scholar
- Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O’Grady NP, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753–71. ArticlePubMedGoogle Scholar
- Dryden MS. Skin and soft tissue infection: microbiology and epidemiology. Int J Antimicrob Agents. 2009;34(Suppl 1):S2-7. ArticleCASPubMedGoogle Scholar
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. ArticleCASPubMedGoogle Scholar
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. ArticleCASPubMedGoogle Scholar
- Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516–8. ArticlePubMedPubMed CentralGoogle Scholar
- George SM, Harrison DA, Welch CA, Nolan KM, Friedmann PS. Dermatological conditions in intensive care: a secondary analysis of the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme database. Crit Care. 2008;12(Suppl 1):S1. ArticlePubMedPubMed CentralGoogle Scholar
- Edelsberg J, Berger A, Weber DJ, Mallick R, Kuznik A, Oster G. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160–9. ArticlePubMedGoogle Scholar
- Zilberberg MD, Shorr AF, Micek ST, Hoban AP, Pham V, Doherty JA, Ramsey AM, Kollef MH. Epidemiology and outcomes of hospitalizations with complicated skin and skin-structure infections: implications of healthcare-associated infection risk factors. Infect Control Hosp Epidemiol. 2009;30(12):1203–10. ArticlePubMedGoogle Scholar
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbachet SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52. ArticlePubMedGoogle Scholar
- Duane TM, Huston JM, Collom M, Beyer A, Parli S, Buckman S, et al. Surgical Infection Society 2020 updated guidelines on the management of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2021;22(4):383–99. ArticleGoogle Scholar
- Sartelli M, Guirao X, Hardcastle TC, Kluger Y, Boermeester MA, Raşaet K, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. ArticlePubMedPubMed CentralGoogle Scholar
- Esposito S, Bassetti M, Concia E, De Simone G, De Rosa FG, Grossi P, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother. 2017;29(4):197–214. ArticleCASPubMedGoogle Scholar
- Rodríguez-Acelas AL, de Abreu AM, Engelman B, Cañon-Montañez W. Risk factors for health care-associated infection in hospitalized adults: Systematic review and meta-analysis. Am J Infect Control. 2017;45(12):e149–56. ArticlePubMedGoogle Scholar
- Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24(1):141–73. ArticleCASPubMedPubMed CentralGoogle Scholar
- Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26(2):185–230. ArticleCASPubMedPubMed CentralGoogle Scholar
- World Health Organization. Prevention of hospital-acquired infections: a practical guide. In Ducel G, Fabry J and Nicolle L editors, 2nd. ed. World Health Organization. (2002). Available at: https://apps.who.int/iris/handle/10665/67350
- Chander Y, Rai R. Hospital Acquired Infection. Med J Armed Forces India. 1998;54(3):179–81. ArticlePubMedPubMed CentralGoogle Scholar
- Griffiths P, Renz A, Hughes J, Rafferty AM. Impact of organisation and management factors on infection control in hospitals: a scoping review. J Hosp Infect. 2009;73(1):1–14. ArticleCASPubMedGoogle Scholar
- Gordts B. Models for the organisation of hospital infection control and prevention programmes. Clin Microbiol Infect. 2005;11(Suppl 1):19–23. ArticlePubMedGoogle Scholar
- O’Boyle C, Jackson M, Henly SJ. Staffing requirements for infection control programs in US health care facilities: Delphi project. Am J Infect Control. 2002;30(6):321–33. ArticlePubMedGoogle Scholar
- Dekker M, van Mansfeld R, Vandenbroucke-Grauls C, de Bruijne M, Jongerden I. Infection control link nurse programs in Dutch acute care hospitals; a mixed-methods study. Antimicrob Resist Infect Control. 2020;9(1):42. ArticlePubMedPubMed CentralGoogle Scholar
- National Action Plan on Antimicrobial Resistance (PNCAR) 2017–2020. Available at: https://www.salute.gov.it/imgs/C_17_opuscoliPoster_362_ulterioriallegati_ulterioreallegato_0_alleg.pdf
- Regione Lazio. Centro regionale rischio clinico. Linee guida per l’elaborazione del Piano Annuale delle Infezioni Correlate all’Assistenza (PAICA) Available at: https://www.regione.lazio.it/sites/default/files/2021-03/Elab-piano-paica-2019.pdf
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, Hooton TM. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121(2):182–205. ArticleCASPubMedGoogle Scholar
- Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, Schwab F, et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect. 2006;64(1):16–22. ArticleCASPubMedGoogle Scholar
- Bootsma MCJ, Diekmann O, Bonten MJM. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci USA. 2006;103(14):5620–5. ArticleCASPubMedPubMed CentralGoogle Scholar
- Chemaly RF, Simmons S, Dale C, Ghantoji SS, Rodriguez M, Gubb J, et al. The role of the healthcare environment in the spread of multidrug-resistant organisms: update on current best practices for containment. Ther Adv Infect Dis. 2014;2(3–4):79–90. PubMedPubMed CentralGoogle Scholar
- European Centre for disease prevention and control 2019. Carbapenem-resistant Enterobacteriaceae. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/carbapenem-resistant-enterobacteriaceae-risk-assessment-rev-2.pdf
- Cantón R. Role of the microbiology laboratory in infectious disease surveillance, alert and response. Clin Microbiol Infect. 2005;11(Suppl 1):3–8. ArticlePubMedPubMed CentralGoogle Scholar
- Garau J, Bassetti M. Role of pharmacists in antimicrobial stewardship programmes. Int J Clin Pharm. 2018;40(5):948–52. ArticlePubMedGoogle Scholar
- Koo E, McNamara S, Lansing B, Olmsted RN, Rye RA, Fitzgerald T, et al. Making infection prevention education interactive can enhance knowledge and improve outcomes: results from the targeted infection prevention (TIP) study. Am J Infect Control. 2016;44(11):1241–6. ArticlePubMedPubMed CentralGoogle Scholar
- Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420–3. ArticlePubMedGoogle Scholar
- Marra AR, Guastelli LR, de Araujo CM, dos Santos JLS, Lamblet LCR, Silva M Jr, et al. Positive deviance: a new strategy for improving hand hygiene compliance. Infect Control Hosp Epidemiol. 2010;31(1):12–20. ArticlePubMedGoogle Scholar
- Sherertz RJ, Ely EW, Westbrook DM, Gledhill KS, Streed SA, Kiger B, et al. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med. 2000;132(8):641–8. ArticleCASPubMedGoogle Scholar
- Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167–73. ArticlePubMedGoogle Scholar
- World Health Organization and WHO Patient Safety. A guide to the implementation of the WHO multimodal hand hygiene improvement strategy. 2009 Available at: https://apps.who.int/iris/handle/10665/70030



